行業資訊
美國開始對中國口罩企業秋后算賬,首家企業面臨1062萬巨額罰款!
對中國口罩企業進行的第一波清算,美國已經開始動手了!
6月5日,美國司法部發布公告,正式起訴第一家中國口罩企業-King Year Packaging and Printing Co. Ltd.(金年包裝印刷有限公司)。
美國司法部給出的起訴理由是,在美國疫情期間,這家中國口罩企業生產并向美國出口將近50萬個假冒偽劣的N95口罩。

▲美國司法部公告
根據美國司法部的公告,這家中國口罩企業面臨三項違反聯邦食品,藥物和化妝品法(FDCA)的指控,每項指控的最高罰款為50萬美元,或者是犯罪所產生的總收入的兩倍或總損失的兩倍(以較高者為準)。
指控如果成立,這家企業將面臨共計至少150萬美元(約合1062萬人民幣)的巨額罰款!

美國起訴書顯示:
從2020年4月6日到2020年4月21日,King Year生產了495,200個假冒偽劣N95口罩,并將這些有缺陷的產品出口到美國。
為了直接吸引了醫護人員和其他消費者,King Year在其呼吸器的包裝上蓋了NIOSH和FDA認證標識,
而實際上,這家中國口罩企業口罩未經NIOSH批準,也未經過FDA授權。雖然口罩上面印有“ N95”,實際檢測出來的過濾效果遠低于N95口罩最低95%的要求。
美國司法部認為這是嚴重的欺騙包括醫護人員和急救人員在內的美國消費者的行為。并使他們處于危險之中。而為了掩蓋其呼吸器質量低下的問題,King Year還對外散布了證明其真實性的虛假文件,并向FDA提交了欺詐性注冊聲明。
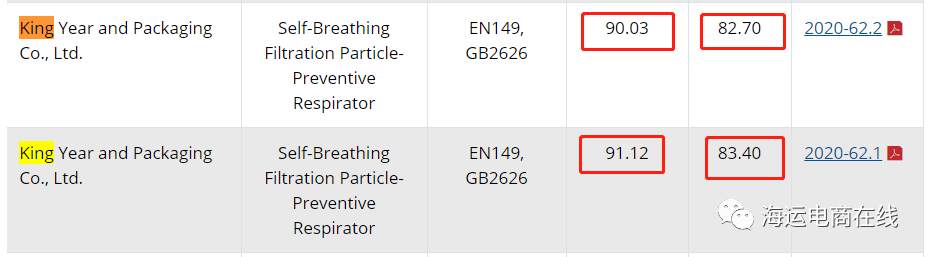
▲CDC檢測結果顯示這家企業過濾值達不到95%這一標準
美國疾病預防與控制中心(CDC)公布的檢測結果顯示,這家中國口罩企業-King Year Packaging and Printing Co. Ltd.(金年包裝印刷有限公司)的根本沒達到N95口罩最低95%過濾效果的技術要求。
以下是美國司法部起訴公告原文:
Chinese Manufacturer Charged with Exporting Misbranded and Defective Masks Falsely Purporting to be N95 Respirators
NEWARK, N.J. – A Chinese manufacturer was charged today with producing and exporting to the United States in the midst of the COVID-19 pandemic nearly half a million misbranded and defective masks that falsely purported to be N95 respirators, U.S. Attorneys Craig Carpenito, District of New Jersey, and Richard P. Donoghue, Eastern District of New York, announced.
King Year Packaging and Printing Co. Ltd. (King Year) is charged by complaint with three counts of violating the Federal Food, Drug and Cosmetic Act (FDCA) for causing misbranded and substandard respirators that falsely purported to meet the N95 standard to be imported into the United States. The complaint also charges the defendant with one felony count of making a false statement by filing misleading registration documents with the U.S. Food and Drug Administration (FDA). The criminal complaint was filed in Brooklyn federal court.
“These charges demonstrate the continued commitment of the Department of Justice and our partners to aggressively pursue those who sell misbranded and defective personal protective equipment, whether they are located here or abroad,” Carpenito said. “We will aggressively investigate and charge manufacturers that put our medical professionals and first responders at risk in fighting this crisis.”
“The charges alleged in this complaint show a blatant disregard for the safety of American citizens,” Acting FBI-Newark Special Agent in Charge Douglas Korneski said. “Had it not been for the actions of the investigative team, this defendant would have put first responders, hospital employees, and other front line workers directly in harm’s way with faulty equipment just to make a buck. The defendant tried to bypass the government's regulations by misbranding the quality of the equipment being peddled. The FBI remains vigilant in the pursuit of criminals trying to exploit the current crisis.”
Attorney General William P. Barr created the COVID-19 Hoarding and Price Gouging Task Force, led by U.S. Attorney Carpenito, who is coordinating efforts with the Antitrust Division and U.S. Attorneys across the country wherever illegal activity involving protective personal equipment occurs. The Secretary of Health and Human Services has issued a notice designating categories of health and medical supplies that must not be hoarded or sold for exorbitant prices.
“U.S. Customs and Border Protection is proud of the expertise we bring to support and assist investigations by our law enforcement partners,” Troy Miller, Director, CBP New York Field Office, said. “It is through interagency partnerships and collaborative efforts, like the one leading to today’s charges that we send a message to foreign manufacturers on the importance of understanding and complying with US health, safety, and import laws.”
“The FDA is actively monitoring the marketplace for fraudulent products related to our battle against COVID-19. The agency will continue to collaborate with our fellow law enforcement partners to bring to justice those who place profits above the public health during this pandemic,” Jeffrey J. Ebersole, Special Agent in Charge, FDA Office of Criminal Investigations’ New York Field Office, said. “Today’s announcement should serve as a reminder that we will take appropriate action against those who jeopardize the health of Americans while taking advantage of a crisis.”
According to the complaint:
From April 6, 2020, to April 21, 2020, King Year manufactured 495,200 defective and misbranded masks that claimed to be N95 respirators, and caused those defective products to be imported into the United States. King Year stamped the NIOSH and FDA logos on the packaging for its respirators, appealing directly to healthcare personnel, when in fact, its respirators were not NIOSH-approved, nor were they approved, cleared, or otherwise authorized by the FDA. King Year’s respirators also were embroidered with “N95,” even though they fell well below the minimum 95 percent filtration standard.
King Year’s misbranded and defective products had the potential to deceive U.S. consumers, including healthcare workers and first responders, into believing they were purchasing authentic N95 respirators, and put them at risk. To cover up the poor quality of its respirators, King Year disseminated false documents attesting to their authenticity and filed a fraudulent registration statement with the FDA.
Each charge carries a maximum fine of $500,000 or the greater of twice the gross gain or twice the gross loss from the offense.
Please report COVID-19 fraud, hoarding or price-gouging to the National Center for Disaster Fraud’s National Hotline at (866) 720-5721, or e-mail: [email protected].
The government is represented by Assistant United States Attorney Jonathan Fayer of the Economic Crimes Unit for the U.S. Attorney’s Office for the District of New Jersey.
The charges in the complaint are merely allegations, and the defendant is presumed innocent unless and until proven guilty.
公告鏈接
https://www.justice.gov/usao-nj/pr/chinese-manufacturer-charged-exporting-misbranded-and-defective-masks-falsely-purporting
50萬個不合格N95面臨被罰1062萬人民幣,這家名為King Year Packaging and Printing Co. Ltd.(金年包裝印刷有限公司)的中國口罩企業,是第一個撞上只是美國清算槍口的倒霉蛋,代表著美國清算行動正式開始。

根據美國對于N95口罩的技術要求,在之前美國疾病預防與控制中心(CDC)官方公布的名單中,最低過濾效果低于95%的中國口罩企業竟然高達七成,這些企業接下來估計要提心吊膽了。
以下是美國疾病預防與控制中心(CDC)公布過濾值達不到95%的口罩企業名單:







檢測名單鏈接:https://www.cdc.gov/niosh/npptl/respirators/testing/NonNIOSHresults.html
美國法律的秋后算賬和官司訴訟,不僅僅是讓你吃了的吐出來,傾家蕩產也是分分鐘的事。
而且,如果口罩生產廠家無法找到,或者口罩標注的生產企業不承認,那么,賣家,外貿出口甚至雙清包稅的物流企業,都有可能陪上身家性命!